

Have you ruled out CTCL?
CTCL incidence is rare—currently estimated to be 6.4 per every 1 million people in the general population.1 As a result, CTCL is likely not top of mind for healthcare providers on a daily basis. When patients are diagnosed and treated early, CTCL may go into long-term remission.2
Cutaneous T-cell lymphomas (CTCL) are characterized by the presence of malignant T cells in chronically inflamed skin lesions3
Mycosis fungoides (MF) is the main manifestation of the disease, making up 54%-72% of all cases3
Early-stage cutaneous T-cell lymphoma often presents like other more common skin conditions. When stubborn cases keep coming back again and again, be sure you have ruled out CTCL.
MF Lesions vs. Other Lesions
MF presents as erythematous skin patches that are often confused for immune-related diseases like eczema, parapsoriasis, psoriasis, or pityriasis lichenoides.4
Notice how MF can present compared to other skin conditions in the photos:
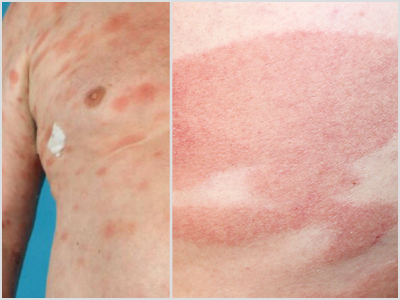
MF | Eczema
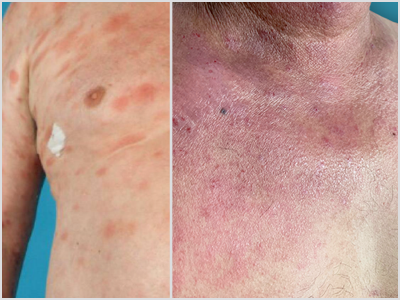
MF | Parapsoriasis
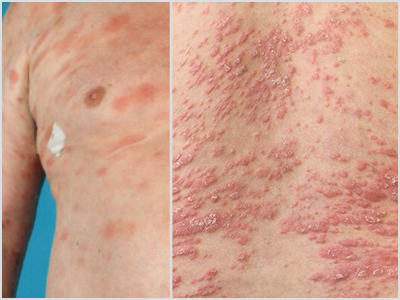
MF | Psoriasis
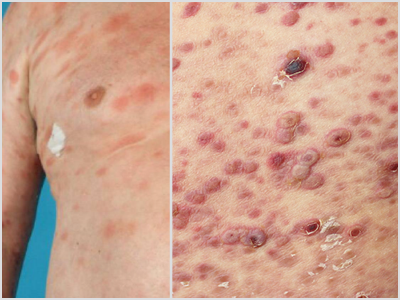
MF | Pityriasis Lichenoides
The following are ways lesions commonly present:
- Patch/plaque lesions often sharply bordered, erythematous, and slightly scaling5
- Lesions tend to persist or recur after treatment for a different skin condition5
- Bathing trunk distribution (upper thigh, buttocks, lower abdomen)5
- More than 80% of people with CTCL report they have itch6
- Lesions may improve with sun exposure5
- Lesions associated with initial phase of mycosis fungoides are nonspecific7
The CTCL Patient Profile
The incidence rate of CTCL tends to be higher among people of certain ages, genders and ethnicities. Considering these factors goes hand in hand with looking for atypical presentation of skin lesions.

Mid-50s
Incidence increases with age, the median age at diagnosis is mid-50s1

2:1 ratio
The disease affects men more often than women1
2x more
Occurs twice as often in people of African-American descent vs. European or Asian descent7

"A case of adult-onset eczema or an asymmetric pattern of disease starting in
Erin Boh, MD, PhD, FAAD
Joseph Chastain Clinical Professor of Dermatology Chair, Department of Dermatology
Tulane University School of Medicine
Have you noticed atypical presentation? Let us help connect you to a specialist for guidance on CTCL identification and treatment.
Diagnosing CTCL—the Malignant Mimicker
CTCL testing and getting an accurate diagnosis often start when a dermatologist reconsiders a patient's current diagnosis and course of treatment.
Early MF is often diagnosed through clinical, histopathologic, and immunopathologic methods including:8
Physical exam: Complete skin examination and palpation of peripheral lymph node regions

Biopsy: Of skin and/or lymph node for pathology

Blood testing: To check for presence of malignant T-cells
Imaging tests: Such as CT or MRI scans
If you have patients presenting with CTCL symptoms or patients who have CTCL, we are here to support you.
Contact us to help you find a local treatment center of excellence, connect you to an expert who specializes in CTCL, and more.
CTCL Treatment and Goals
Most treatments do not result in durable remissions off of treatment and are not given with curative intent. An important part of disease and symptom control is to minimize the risk of infections and treat pruritus.
Skin-directed therapies are often used for the treatment of early-stage CTCL, including:9
- Topical mechlorethamine
- Topical retinoids (Bexarotene, Tazarotene)
- Topical corticosteroids
- Phototherapy
- Topical imiquimod
- Topical carmustine
Such therapies are appropriate for early-stage disease confined to the skin and are often used in combination with systemic therapies for advanced-stage disease.1
Targretin Gel for Early-Stage CTCL

Ortho Dermatologics is the marketer and distributor of TARGRETIN (bexarotene) 1% gel, a skin-directed therapy for early-stage CTCL patients who:10
- Have refractory or persistent disease
- Or have not tolerated other therapies
Bexarotene is in a subclass of retinoids (rexinoids) that selectively activate retinoid X receptors (RXRs). These retinoid receptors have biologic activity distinct from that of retinoic acid receptors (RARs).10
Scroll down for Important Safety Information.

Bexarotene selectively activates retinoid X receptors.

Activated receptors regulate gene expression in the cell.

Tumor growth is inhibited and regression is induced.
The exact mechanism of action of bexarotene in the treatment of CTCL is not known.
Patient Convenience
Targretin Gel has minimal application hazards, making it easy for patients to start and stay on their treatment plans.10

Noncytotoxic

No refrigeration required

No gloves required
Demonstrated Efficacy
In a Phase 3 Trial, Targretin Gel produced an overall response rate of 26% (13/50) after 16 weeks of treatment by the Composite Assessment of Index Lesion Severity.10

Responses were seen as early as 4 weeks after starting treatment with Targretin Gel10

The median time to best response was 85 days after starting treatment10
Find more information in the National Comprehensive Cancer Network Guidelines for Primary Cutaneous Lymphomas.
Get details on Targretin Gel's pharmacokinetics, dosing, clinical studies, and more on the official HCP site. There, you can also learn about Targretin Capsules for later-stage CTCL treatment.
Helping Patients Access Targretin Gel


Most eligible commercially insured patients may pay as little as $0 on their Targretin prescription.*
*Terms, conditions, and limitations apply.
Visit www.targretin.com for eligibility criteria and terms and conditions.
Expand to see more
INDICATION AND USAGE
Targretin (bexarotene) gel 1% is indicated for the topical treatment of cutaneous manifestations of cutaneous T-cell lymphoma (Stages 1A and 1B) in patients who have refractory or persistent disease after other therapies or who have not tolerated other therapies.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
- Targretin gel is contraindicated in patients with a known hypersensitivity to bexarotene or any of its components and should be used with caution in patients with a known hypersensitivity to other retinoids.
- Targretin gel may cause fetal harm when administered to a pregnant woman. Targretin gel must not be given to a pregnant woman or a woman who intends tobecome pregnant. If a woman becomes pregnant while taking Targretin gel, Targretin gel must be stopped immediately and the woman should be given appropriate counseling.
- To prevent pregnancy, effective contraception must be used for one month prior to the initiation of Targretin gel therapy, during therapy and for at least one month following discontinuation of therapy; it is recommended that two reliable forms of contraception be used simultaneously unless abstinence is the chosen method. Male patients with sexual partners who are pregnant, possibly pregnant or who could become pregnant must use condoms during treatment and for at least one month after the last dose of drug.
- No more than a one month supply of Targretin gel should be given to the patient so that the results of pregnancy testing can be assessed and counseling regardingavoidance of pregnancy and birth defects can be reinforced.
PRECAUTIONS
- Because of the relationship of bexarotene to Vitamin A, patients should be advised to limit Vitamin A supplements to avoid potential additive toxic effects.
- Patients should be advised to minimize exposure to sunlight and artificial ultraviolet light during the use of Targretin gel.
- Patients who are applying Targretin gel should not concurrently use products that contain DEET, a common component of insect repellent products.
- Due to the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
- The most common adverse events reported in Targretin gel clinical trials with an incidence at the application site of at least 10% were: rash, pruritus, skin disorder, and pain.
Please click here for full Prescribing Information.
1. Akilov OE. National Organization for Rare Disorders website. www.rarediseases.org/physician-guide/cutaneous-t-cell-lymphomas-ctcl. Accessed March 24, 2021. 2. Cutaneous Lymphoma Foundation website. www.clfoundation.org/cutaneous-t-cell-lymphoma. Accessed February 4, 2021. 3. Krejsgaard T, Lindahl LM, Mongan NP, et al. Malignant inflammation in cutaneous T-cell lymphoma—a hostile takeover. Semin Immunopathol. 2017;39(3):269-282. 4. Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115(4):798-812. 5. Cutaneous Lymphoma Foundation. Cutaneous Lymphomas Diagnosis and Staging [Video] Available at www.clfoundation.org/cutaneous-lymphomas-diagnosis-and-staging-0. Accessed February 1, 2021. 6. Cutaneous Lymphoma Foundation. www.clfoundation.org. Accessed February 1, 2021. 7. National Organization for Rare Disorders. Rare Disease Database: Cutaneous T-Cell Lymphomas. www.rarediseases.org/rare-diseases/cutaneous-t-celllymphomas/. Accessed February 1, 2021. 8. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): T-Cell Lymphomas. Version 5.2018. Published August 13, 2018. 9. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Primary Cutaneous Lymphomas. Version 2.2019. Published December 17, 2018. 10. Targretin gel 1% [prescribing information]. Bridgewater, NJ: Bausch Health US, LLC.